Formula For Acetylene Gas
Formula For Acetylene Gas. Acetylene is capable of producing the hottest flame of all other gases. A combustion reaction occurs when a compound merges with oxygen to release heat in an exothermic reaction.

 1. Acetylene (C2H2) undergoes combustion in excess oxygen from www.homeworklib.com
1. Acetylene (C2H2) undergoes combustion in excess oxygen from www.homeworklib.comAcetylene is a fuel gas (c 2 h 2) composed of carbon and hydrogen. It is a colourless, inflammable gas widely used as a fuel in oxyacetylene welding and cutting of metals and as raw material in the synthesis of many organic chemicals and plastics;. (b) how many moles of each product would form?

In the reaction 2 h2c2 + 5 o2 ? For acetylene, the carbon yield has been found to be in the range of 65 percent or greater (refs.
Acetylene is called d.a.— dissolved acetylene. It is produced when calcium carbide comes in contact with water.
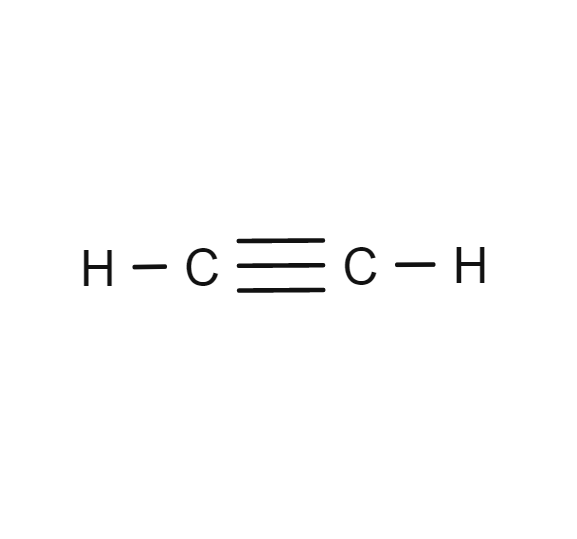
A colorless gas with slight solubility in water, acetylene has a distinct odor. In addition, this molecule is the simplest alkyne, which is a functional group that scientists characterize by having triple bonds.

This method is used for cutting or welding materials which require temperatures of up to 3,500 ° c (6,330 ° f). Its molecular weight is 26.04g/mol.
It is produced when calcium carbide comes in contact with water. Acetylene = c 2 h 2.

Acetylene gas, c2h2, is produced as a result of the following reaction: Acetylene and other alkynes are generally prepared from calcium carbide, vicinal dihalides or by.
Oxygen (commercial) is contained in a black coloured cylinder. Each molecule of this hydrocarbon compound contains two carbon atoms and two hydrogen atoms.

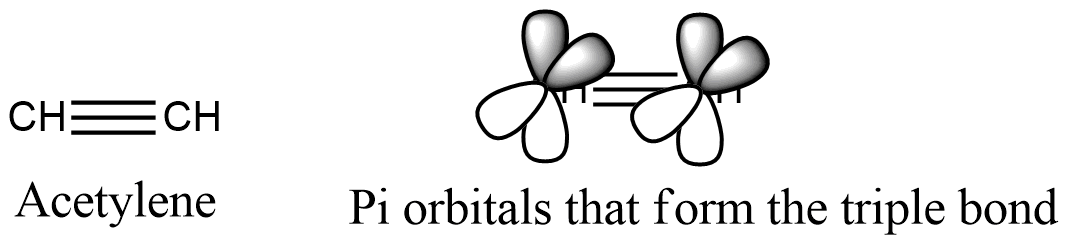
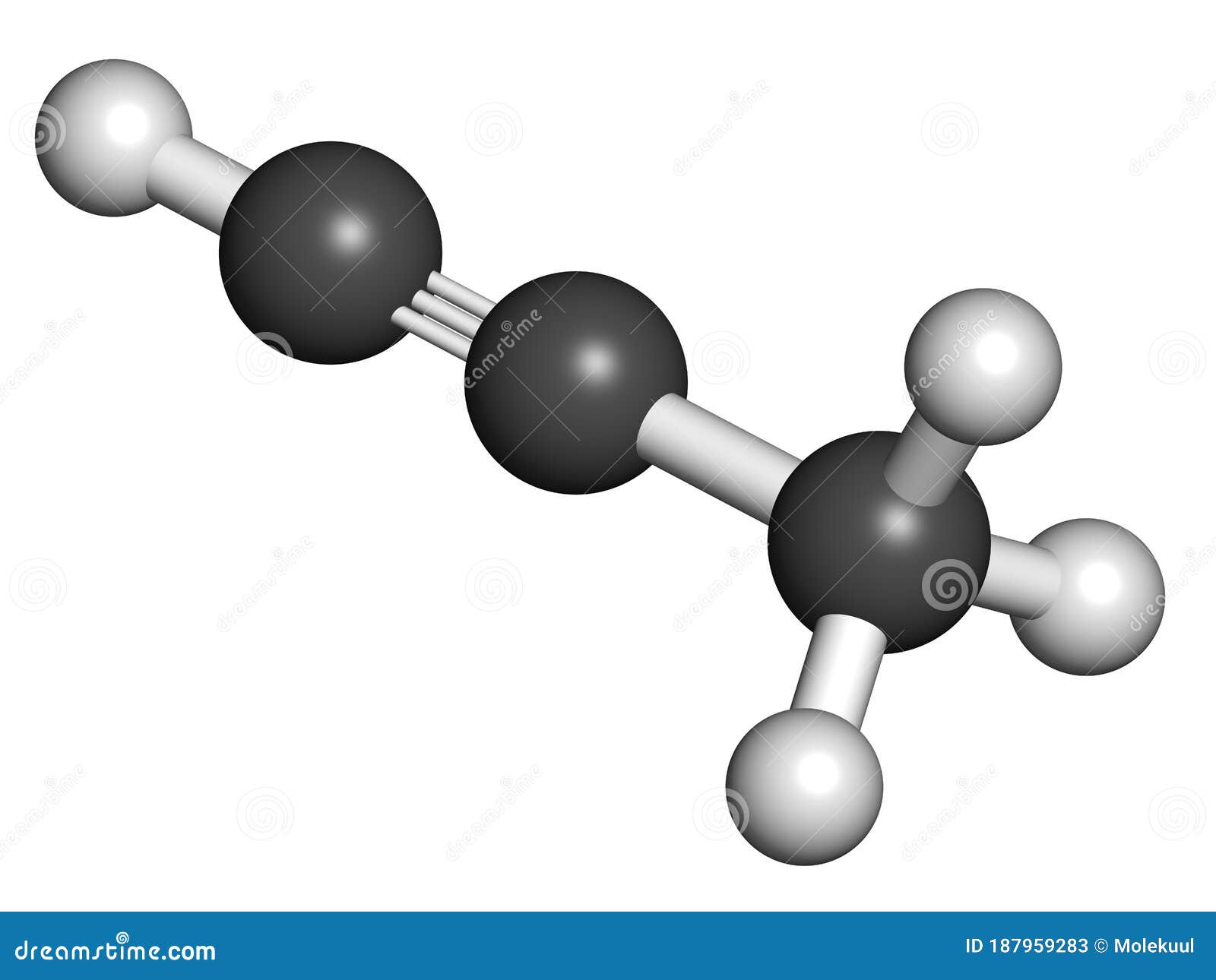
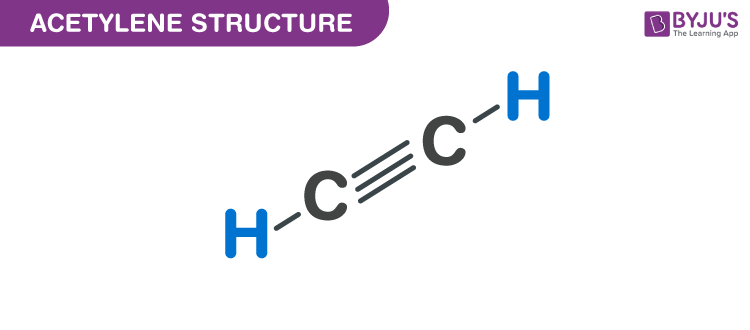
Acetylene has a triple bond between atoms of carbon. Its chemical symbol is c 2 h 2.

The chemical compound can be produced using several methods the most commonly used method is the hydrolysis of calcium carbide. It is an unsaturated hydrocarbon and the simplest alkyne.
The two carbon atoms are held together by what is known as a triple carbon bond having ch bond angle of 180 deg. Acetylene has a triple bond between atoms of carbon.
Its chemical symbol is c 2 h 2. 3) acetylene gas is formed by the mixture calcium carbide and water and is composed of carbon and hydrogen having the chemical formula c.
(a) if 32 grams of cac2 are consumed in this reaction how many soft water should be needed? Acetylene is a hydrocarbon consisting of two carbon atoms and two hydrogen atoms.
It is an unsaturated hydrocarbon and the simplest alkyne. It is a colourless, inflammable gas widely used as a fuel in oxyacetylene welding and cutting of metals and as raw material in the synthesis of many organic chemicals and plastics;.
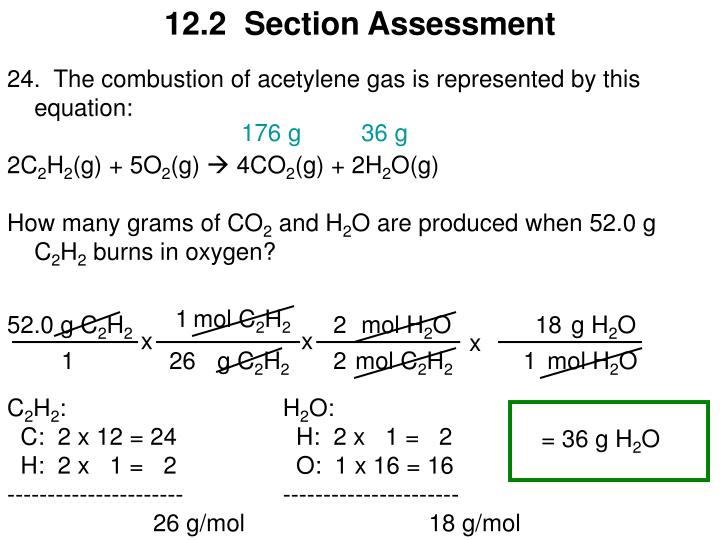
Acetylene is called d.a.— dissolved acetylene. Acetylene gas, c2h2, is produced as a result of the following reaction:

Acetylene gas, c2h2, is produced as a result of the following reaction: Acetylene and other alkynes are generally prepared from calcium carbide, vicinal dihalides or by.

Acetylene or ethyne is a colorless gas with the general molecular formula c 2 h 2 when combustion with oxygen to form a luminous smoky flame due to the high carbon materials. The orientation is in the same straight line and the bond angle is 180 degrees.

A combustion reaction occurs when a compound merges with oxygen to release heat in an exothermic reaction. Acetylene gas, c2h2, is produced as a result of the following reaction:

Acetylene and several other names) is c2h2. Today, acetylene is also used in the production of several plastics.
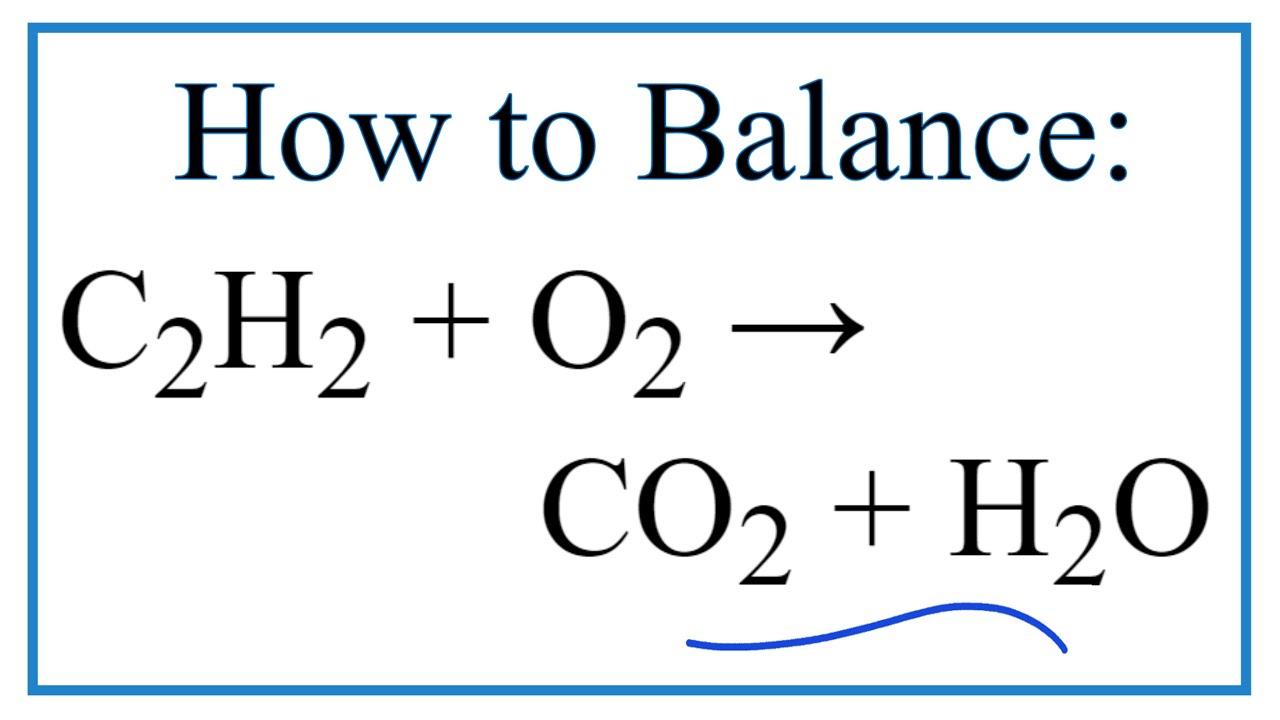
Acetylene is a fuel gas (c 2 h 2) composed of carbon and hydrogen. Alkynes contain four hydrogen atoms less than corresponding alkanes and two hydrogen atoms less than corresponding alkenes and have the general formula c n h 2 n − 2.

It is an unsaturated hydrocarbon and the simplest alkyne. Acetylene or ethyne is a colorless gas with the general molecular formula c 2 h 2 when combustion with oxygen to form a luminous smoky flame due to the high carbon materials.
The Acetylene Chemical Formula Is C 2 H 2 And Its Extended Formula Is Chξch.Now a known volume of water is added to the conical ask containing calcium carbide in turn producing acetylene gas. An acetylene molecule is composed of two carbon atoms and two hydrogen atoms. Availability from the carburizing gas that is used.
Acetylene Is A Fuel Gas (C 2 H 2) Composed Of Carbon And Hydrogen.3) acetylene gas is formed by the mixture calcium carbide and water and is composed of carbon and hydrogen having the chemical formula c. When acetylene is passed through it, the water is displaced inside the measuring cylinder and the volume of the gas is obtained. The acetylene formula (the empirical formula of acetylene) can be represented as c2h2.
A Combustion Reaction Occurs When A Compound Merges With Oxygen To Release Heat In An Exothermic Reaction.If released to air, a vapor pressure of 3.65x10+4 mm hg at 25 °c indicates acetylene will exist solely as a gas in the atmosphere. Acetylene has a triple bond between atoms of carbon. The acetylene gas so formed travels through the flexible tube and enters the measuring tank.
An Acetylene Molecule Has Two Carbon Atoms Bonded By A Triple Bond And Two Hydrogen Atoms.Acetylene is used in welding and cutting processes. Acetylene is a hydrocarbon consisting of two carbon atoms and two hydrogen atoms. Acetylene = c 2 h 2.
Its Chemical Formula Is C 2 H 2, And The Extended Formula Is Ch≡Ch.Oxygen (commercial) is contained in a black coloured cylinder. (b) how many moles of each product would form? For commercial purposes, acetylene can be made from several different raw materials depending on the process used.
Belum ada Komentar untuk "Formula For Acetylene Gas"
Posting Komentar